Request a quote for an onsite or online virtual course for an agreed number of delegates from your site. Whilst course started out being heavily focused as a GAMP 5 Training Course the scope and application has been broadened.
The GAMP 5 3 Day Training Course can be delivered in a hotel one of our offices your premises or online via a virtual classroom.

Gamp 5 online training. The basis of the Live Online Training will be the current requirements for the validation of computerised systems like GAMP and their GxP-oriented application in practice. This fundamental course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and explores tried tested. The development of the GAMP 5 risk man-agement approach has its antecedents in the FMEA-based risk assessment tool published in GAMP 4 in 2001.
While theres nothing that requires this structure or these titles its helpful to see the breadth of involvement in validation. The course content can be tailored to meet your requirements. GAMP 5 provides pragmatic and practical industry guidance to achieve compliant computerized systems fit for intended use in an efficient and effective manner.
This online training course includes the new revised EU GMP Annex 11 and an update on 21 CFR Part 11. Understanding Validation GAMP 5 21 CFR Part 11 and Data Integrity Training Programme. A Risk Based Approach to GxP Process Control Systems may be applied to achieve process control systems that are fit for intended use and meet current regulatory requirements.
The main roles and responsibilities include. The approach matured in the 2005 ISPE GAMP Good Practice Guide. Request a quote for an onsite or online virtual course for an agreed number of delegates from your site.
The Understanding Validation GAMP 5 21 CFR Part 11 and Data Integrity 3 Day Training Programme provides learners with the opportunity to attend one course alone a mix of the following courses or all three in their entirety over three consecutive days. GAMP 5 Risk Management Approach This GAMP5 Risk Management Approach training course is a 1 Day public programme designed to guide participants through the latest GAMP Guidance and give them a good understanding of the current best practices for GxP compliant computerised systems in regulated environments. Experts from the pharmaceutical industry and from the GAMP Committee will show you efficient ways to.
David Thompson Managing Director and Principal Consultant at Clarity Compliance Solutions is a. Takes place on 2 nd 4 th June 2020. GAMP 5 defines a set of fairly typical roles in validation.
This fundamental course introduces participants to regulatory requirements for computerized systems in the pharmaceutical industry and explores tried. The course covers recommended good practice based on a life cycle approach for the development. Basic Principles of Computerized Systems Compliance Using GAMP 5 Including Revised Annex 11 and Part 11 Update T45 - Updated.
GAMP 5 GxP Process Control Training Course. This highly interactive course describes how the GAMP Good Practice Guide. This computerised systems validation training course covers the essential principles on how to use a risk-based approach in Computerised Systems Validation CSV.
A Risk-Based Approach to Compliant Electronic Records and Signatures with incorporation of aspects of ISO 14971 Medical Devices Appli-. GAMP Computerised Systems Validation Training Virtual This training course on how to validate computerised systems covers the essential principles on how to use a risk-based approach in Computer Systems Validation CSV. - The GAMP 5 Approach Live Online Training on 17 November and 18-20 November 2020 23 March 2021 and 24-26 March 2021 Including implications of EU GMP Annex 11 computerised systems GMP Certification Programme Certified Computer Validation Manager.
The course content can be tailored to meet your requirements. The GAMP 5 Training Course can be delivered in a hotel one of our offices your premises or online via a virtual classroom. This technical document describes a flexible risk-based approach to compliant GxP regulated computerized systems based on scalable specification and verification.
They not only know the industry incredibly well but several of them have been heavily involved in the development of GAMP and associated special interest group guidance. This course offers a high level compliance review of the. Our training course tutors have a wealth of experience.
The course provides a broad overview of GMP requirements for computerised systems and is designed for professionals seeking training on a practical.

Gamp Training Courses Ispe International Society For Pharmaceutical Engineering

Intland Software Announces Pharma Gamp 5 Validation Quality Risk Management Template Intland Software

Accelerate Digital Pharma Systems Validation

Codebeamer X Gamp 5 Template Introduction Intland Software

Live Online Training Computerised System Validation The Gamp 5 Approach Eca Academy

Computer Validation The Gamp 5 Approach Eca Academy

Key Principles Of Gamp 5 For Computer System Validation Youtube

Gamp 5 Guide Compliant Gxp Computerized Systems Ispe International Society For Pharmaceutical Engineering
Achieving Maintaining Gamp 5 Compliance Risk Based Approach To Software Development Verification Pharmaceutical Engineering
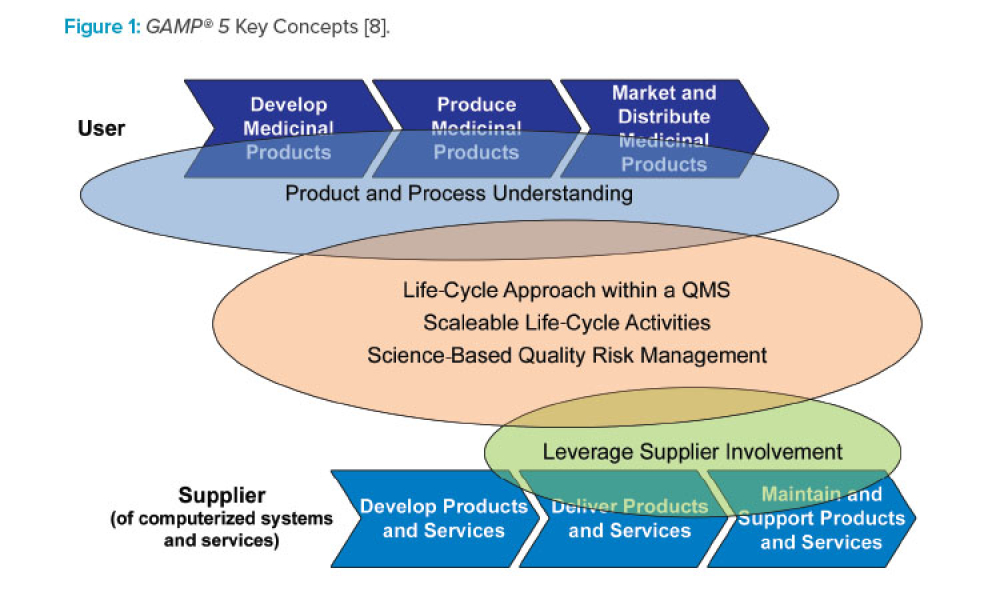
Gamp Resources Ispe International Society For Pharmaceutical Engineering

Webinar Analytical Instrument Qualification Gamp Regulations For Computer System Validation Data Integrity Eca Academy

Gamp 5 Innovation In A Flexible Manner Learnaboutgmp Accredited Online Life Science Training Courses




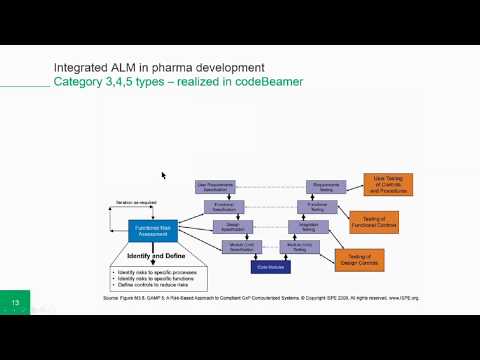
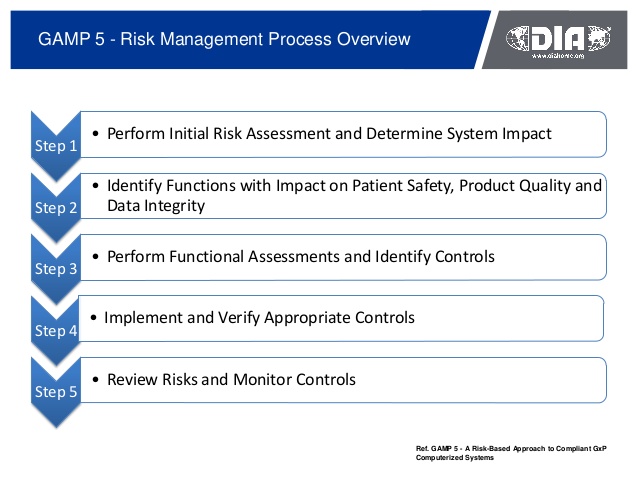


0 comments